Soil and water quality are vital ingredient for any successful aquaculture practices. Even though these problems are caused by the way the land is set up, some soils have bad qualities like acid sulphate, high organic content, and too much porosity.
In the same way, the water may be of poor quality, like being very acidic, full of nutrients and organic matter, full of suspended solids, or contaminated with chemicals from industry or agriculture.
1. Soil Quality Parameters
People think of a pond’s soil as its chemical lab. In pond farms, the quality of the soil is important not only because it affects the yield and quality of the water above it, but also because it can be used to build a dike.

The way the soil is made also has a big effect on how well the pond can keep the right amount of water in it. Soil quality takes into account how the soil’s physical, chemical, and biological parts work together.
Aquaculture, on the other hand, has a lot of problems with soil quality, so there are many ways to make pond soils better.
# Physical Parameters:
Texture of the soil
Texture of the soil: The best soil for a pond shouldn’t be too sandy, which would let the nutrients run off, or too clayey, which would keep all the nutrients on the soil. Touch and feel can give you a good idea of the texture.
Below, you can find the size limits and some general traits of the soil’s parts.
Soil Structure and Macropores
Soil structure is how the primary soil particles are put together or set up into aggregates. It changes how water and air move through the soil, which has a big effect on the soil’s ability to support life and do other important things. Pores are spaces between and inside of aggregates that are filled with water and air.
Macropores are large holes in the soil that are usually bigger than 0.08 mm in diameter. They are usually found between soil particles. They give soil organisms a place to live, and plant roots can grow into them.
Micropores are small soil pores with diameters of less than 0.08 mm. They are usually found in structural aggregates. To get water out of micropores, you need suction.
Aggregate Stability
Aggregate stability is the ability of soil particles to stay together when forces like tillage, water erosion, or wind erosion try to break them apart. The size distribution of dry aggregates can be used to predict how well a soil will resist abrasion and wind erosion.
Available Water Capacity
Available Water Capacity: The maximum amount of water that plants can get from the soil is called its “available water capacity.” It shows how well a soil can hold on to water and make it available for plants to use.
Bulk Density
Bulk Density: It is found by dividing the dry weight of the soil by the amount of soil. This volume includes both the size of the soil particles and the size of the spaces between them. Most of the time, bulk density is measured in g/cm3.
Infiltration
Infiltration is the process of water going down into the soil. Infiltration rate is the speed at which water moves into the soil. Most of the time, the rate of infiltration is measured in inches per hour. For water from rain or irrigation to be useful, it must first get into the soil.
Slaking
Slaking is when large, air-dry soil aggregates (>2-5 mm) break down into smaller micro-aggregates (0.25 mm) when they are suddenly put into water. Slaking happens when aggregates aren’t strong enough to handle the stress that comes from taking in water quickly.
Internal stresses happen when clay particles swell differently, when air gets stuck in soil pores and can’t get out, when heat is released quickly when the soil gets wet, and when water moves through the soil.
Soil Crusts
Soil Crusts: There are also biological and chemical factors that can lead to soil crusting. A biological crust is made up of living Lichen, Cyanobacteria, Algae, and Moss that grow on the top of the soil and hold it together. Soils with a lot of salt can form a chemical crust where the salt has settled out.
# Chemical Parameters:
Soil pH
Soil pH: More specifically, it is a measure of how many hydrogen ions are in a solution of water. Soil pH ranges from 3.5 (very acid) to 9.5. (very alkaline). Soil pH should be between 6 and 8, but it can be acidic, alkaline, or neutral.
Fertilization doesn’t work well in acidic ponds, and liming is the only way to improve the quality of water in acidic soil.
Soil salinity
Soil salinity: High rates of evaporation and low annual rainfall can cause salts to build up in irrigated soils, which could be a problem. Salts can come from water used for irrigation, fertilizers, compost, and even manure. Salts can be taken away by slowly pouring on too much water.
Cation Exchange Capacity (CEC)
A cation is an ion with a positive charge. Ca2+, Mg2+, K+, NH4+, Zn2+, Cu2+, and Mn2+ are all cations. These cations are in the soil solution and are in a dynamic balance with the cations that stick to clay and organic matter.
CEC is a way to measure how many cations a soil can hold and hold on to. CEC depends on the types and amounts of organic matter (OM) and clay in the soil. In general, the CEC goes up with the amount of OM and clay.
Plant Nutrients
These things can be put into two different groups: macronutrients and micronutrients. Carbon (C), Oxygen (O), Hydrogen (H), Nitrogen (N), Phosphorus (P), Potassium (K), Calcium (Ca), Magnesium (Mg), and Sulfur (S) are all macronutrients.
These are the most important nutrients for plant growth, so plants need a lot of them. The micronutrients, like iron (Fe), zinc (Zn), manganese (Mn), boron (B), copper (Cu), molybdenum (Mo), and chlorine (Cl), are needed in smaller amounts but are still important for plant growth and development (Cl).
Almost all nutrients are taken up by plants from the soil solution as cations or anions, which are ionic forms.
Calcium Carbonate
Calcium Carbonate: Calcium carbonate in moderate amounts is good for the structure of the soil and is often used to raise the pH of acidic soils.
However, when the amount of calcium in the soil is higher than what the soil can absorb, it binds with other elements and forms compounds that are hard for plants to take in.
Too much calcium can make it harder for plants to get phosphorous, boron, and iron.
# Biological Parameters:
Soil Organic Matter (SOM)
How SOM makes soil properties better? Physically, it stabilizes the structure of the soil, makes it better at holding water, makes it less dense, and its dark color may change its thermal properties.
Chemical
Higher CEC, acts as a pH buffer, binds up metals, and interacts with xenobiotics.
Biological
It gives soil organisms energy and body-building materials, increases the number of microbes and their activities, acts as a source and sink for nutrients, makes ecosystems more resilient, and changes the enzymes in the soil.
About 1% to 4% of the nutrients in the organic matter of the soil are made available to plants each year by the changes made by microbes. Release is fastest when it is warm and wet and slowest when it is cool and dry. Plants get nutrients from the soil because of microorganisms.
Respiration
Soil respiration is the process by which CO2 is released from the surface of the soil.
This CO2 comes from a number of places, including aerobic microbial decomposition of soil organic matter (SOM) to get energy for growth and function (microbial respiration), plant root and animal respiration, and, eventually, the dissolution of carbonates in soil solution.
Soil Enzymes
Soil enzymes speed up the rate at which plant waste breaks down and releases nutrients that plants can use. Soil enzymes come from living and dead microbes, plant roots and waste, and animals that live in the soil.
Microorganisms in soil
Microorganisms make up less than 0.5% (w/w) of the mass of the soil, but they have a big effect on how the soil works and what it does.
The microflora are responsible for 60–80% of the total soil metabolism. Soil microorganisms have more of them than any other kind of organism.
- One gram of topsoil can have up to a billion bacteria.
- Actinomycetes: up to 100 million.
- One million fungi.
- 100 nematodes.
Why soil microorganisms are important?
- They are in charge of recycling C, N, and other nutrients.
- Enhance soil structure.
- Move organic materials and let them break down.
- Keep the soil healthy and good.
- Increase how much air and water can get into the soil.
- Involved in the spread and control of diseases.
2. Water Quality Parameters
Water Quality Parameters: Fish are more likely to get sick and are hard to control. The balance between disease, the environment, and the health of fish is important.

If the balance changes, fish become stressed and more likely to get sick, which can affect their ability to grow and live.
There are physical, chemical, and biological parameters that describe the quality of water.
# Physical Parameters:
Pond Soil
Most of the time, a pond built on land that isn’t good for farming and doesn’t get any nutrients from outside sources is also not good for farming. A pond is good for farming where the soil is good for farming.
If you want to build a productive pond, loamy soil is a good choice. Clay-loam and sandy-clay are also good for making ponds. Clay or sandy soil, on the other hand, is not good for building ponds.
A pond shouldn’t be built on acidic soil where organic matter is breaking down.
Pond Depth
The depth of the pond also affects how many fish are made. The pond shouldn’t be less than 1 m deep or more than 5 m deep; 2 m is the best depth where there is a regular water supply.
Light
How much and how brightly light can get into a body of water depends on its surroundings, such as the condition of the bank, the season, the time of day, the aquatic plants, the amount of turbidity, its location, etc.
Making it easier for light to get into the water of the pond will help with both primary productivity and fish production.
Temperature
Metabolism and biochemical reactions happen at a certain rate because of the temperature. Fish are poikilothermic, which means that the temperature controls their growth, reproduction, and other bodily functions.
When the temperature goes up by 1 C, fish metabolism goes up by 10%. In the same way, a 10 C rise in temperature causes the metabolic rate to double.

Temperature has a lot to do with how much solar radiation gets into the water. It depends on the amount of sunlight, the season, the time of day, the location, the depth of the water, the weather, and other things.
When it is very cold, fish can’t grow, and when it is very hot, they will die. Fish grow all year round in Bangladesh, and very low temperatures don’t hurt them. However, very high temperatures can kill fish, especially in shallow, cloudy water.
During the very hot summer, fish in shallow ponds can be kept safe from high temperatures by keeping aquatic weeds that float or spread in the ponds.
Temperature can be set to the best level in controlled environments like hatcheries, but it’s hard to do so in big bodies of water. The operation of an aerator helps break up the thermal layering, and planting trees gives shade.
Turbidity
Turbidity is a very important thing to think about when raising fish. The amount of turbidity depends on things like the type of soil, the amount of phytoplankton, the season, the amount of surface runoff, and so on. It can be measured by looking at where the best visibility is, which is between 40 and 60 cm.
Turbidity happens when there are too many phytoplankton for fish farming and for other reasons. This limits primary productivity by blocking sunlight and making it hard for fish to breathe.
It can be stopped by putting 500–1000 kg/ha of organic manure on the land and spraying 150–200 kg/acre of hydrated lime Ca(OH)2 in the pond mixed with water. 20–60 kg/acre pond can be used of commercial alum crystal.
# Chemicals Parameters:
Dissolved Oxygen
The atmosphere and photosynthesis are the two ways that oxygen gets into water. Oxygen gets used up in a body of water when organisms breathe, when organic matter breaks down, when phytoplankton blooms, when it’s cloudy, when underground water is used, when the temperature goes up, when there’s turbidity or iron in the water, etc.
If there isn’t enough oxygen in the water, fish may die. When there isn’t enough dissolved oxygen (less than 3ppm), fish become restless, move in strange ways, and gulp at the surface. A fish that died from not getting enough oxygen was found with its mouth open.
Dissolved oxygen levels of 3ppm or less are considered dangerous or even deadly, and freshwater fish should be able to live in water with 5ppm or more of dissolved oxygen or more.
Aeration has been shown to increase the amount of DO that is available. To get dissolved oxygen into the water in an emergency, you can stir up the surface water with bamboo poles or swim. To make oxygen available in water, potassium permanganate can be sprayed at a level of 1-3ppm.
Carbon dioxide
CO2 comes from the air (0.03%), organisms that breathe, bacteria that break down, and other things. Carbon dioxide levels of more than 20 parts per million (ppm) may be bad for fish, and concentrations of less than 3–5 ppm of dissolved oxygen may be just as bad.
Hydrogen Sulfide
It is made when bacteria break down organic matter in an environment with no oxygen. It is usually found at the bottom, where dead organic matter breaks down quickly.
A fresh water fish pond shouldn’t have any hydrogen sulfide in it because fish lose their balance at a level of 0.01ppm. By changing the water often and raising the pH with lime, its toxicity can be lessened.
Ammonia
It is made when protein is broken down by bacteria. It can also be made when some bacteria, molds, and blue-green algae fix N2. If there is more than 12 ppm of ammonia, it could hurt or kill fish. When water mixes with ammonia (NH3), it makes NH4OH.
Ammonia in the form of NH4OH is toxic to fish, but ammonia in the form of NH4+ is not. Salt added at a rate of 1200–1800 kg/ha lowers toxicity.
Through the nitrification process, a biological filter can be used to clean water by changing harmful ammonia into harmless nitrate.
Methane
Most of the time, it doesn’t hurt fish, but if there is a lot of it, it could hurt them.
Nitrogen
Nitrogen is a gas that doesn’t do anything in chemistry or biology. 78% of the air is made up of nitrogen. Nitrogen in the air dissolves in water, and it can also be made by removing nitrogen from ammonia.
Gas bubble diseases can be caused by an unusually high amount of nitrogen. The bad effects of toxic things like ammonia, methane, and so on can be lessened by draining the pond every three years.
pH
pH is a measure of how acidic or alkaline a body of water is. It is the negative logarithm of the number of hydrogen ions. Between 6.5 and 9 is a good pH range for raising fish.
When the pH is less than 6.5, fish don’t grow as fast, their bodies don’t work as well, and they can’t handle toxic substances as well.
Fish get parasites and other diseases when the pH is low, and fish also die when the pH is 11.
Total Alkalinity
Most of the time, alkalinity refers to how much carbonate, bicarbonate, and hydroxide ions are in water. For fish to grow in ponds, the total alkalinity should be more than 20 parts per million.
Total Hardness
Total Hardness: Fish can be raised in water with a total hardness of more than 15 ppm. If the hardness is less than 5 ppm, it can hurt or kill fish. By liming regularly, you can keep the total alkalinity and hardness of the soil.
# Biological Parameters:
Indicator Microorganisms
Indicator Microorganisms: The more coliform bacteria there are, the more likely it is that there are pathogens in the water. Because pathogens are hard to spot, indicator organisms are used instead.
Primary Productivity
Productivity is the rate at which organic matter is made by a producer through photosynthesis in an ecosystem. In a natural setting, fish production is directly linked to primary production. Primary production is also important for the production of zooplankton.
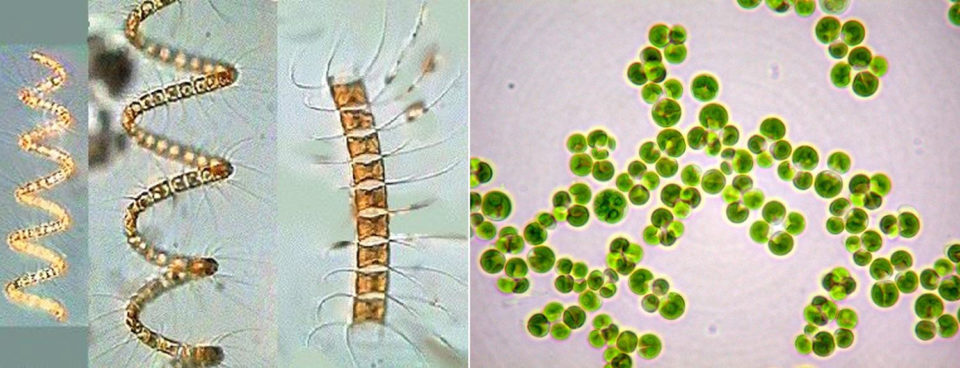
Plankton, which includes phytoplankton and zooplankton, is a small plant or animal, usually microscopic, that drifts in water because of waves, currents, and other movements.
Phytoplankton includes things like Chlorella, Volvox, Anabaena, Microcystis, etc.
Zooplankton – Daphnia, Moina, Bosnia, Sida etc.
Benthos – All of the aquatic organisms that live at the bottom of a pond are called benthos. For example, chironomid larvae are benthos.
The production of phytoplankton, zooplankton, and benthos in their natural state is very important to the production of fish.